6/20/09
...TiAda MusTahilll...
MESIR menang ke atas juara dunia ITALY....MESIR hampir memalukn BRAZIL bekas juara dunia dgn jaringan penalti yg menyelamatkn juara dunia....
IRAQ hampir menjatuhkn maruah SEPANYOL bekas juara dunia......
Tgk bknkah pelik tp x mustahil utk pasukan kerdil mcm MALAYSIA utk menjuarai PIALA DUNIA suatu hari nati...walopon KALAH ngn CHELSEA sebuah kelab je(kelab pn x bleh lawan huhuh)..jgn gelak...jgn ketawa...tp ak pon gelak jgk nie hehehehe....tp tgk la nati...tiade mustahil didunia nie erkkkk......so yg dpt rndah result last sem tu ESPECIALLY AKu huhuhu anda sume maybe akn dpt DEKAN sem dpn nati....jgn gelak....jgn mengilai.....tp kena usaha la...jgn duk jd MAT JENING je...asyik duk tggu pastu mula la nk stress2 bila dpt result...chaiyookkk...
....sem dpn DEKAN sume kengkawan.......
for yg berjaya tu,,, CONGRATZZZ...
6/19/09
Oh My Housemate......
hahahaha ak x sabo nk mkn RFC free nie... bila nk blajo ak MY HOUSMEATE.
kepingin bangat nk mkn. ye la free katekn hahaha..
kalo x pn 2 pot sizzling ok jgk kaloo x nk p sg.petani tu yg pntg FREE heheheheh.BEST2...........
KPD My Housemate yg sorang ag tu SORY bebyk.. ak taw ak x seperfect yg ko maw kn... ko je la yg perfect, ak je yg bangang huhuh. ak sedar sape ak huhuhuhuh...
tp THANX for everything...
Bila nk main bdminton hah???jom r, ajok ramai2 hahaha
6/17/09
RATES OF REACTIONS
The rate of a reaction is the speed at which a reaction happens. If a reaction has a low rate, that means the molecules combine at a slower speed than a reaction with a high rate. Some reactions take hundreds, maybe even thousands of years while other can happen in less than one second. The rate of reaction depends on the type of molecules that are combining.
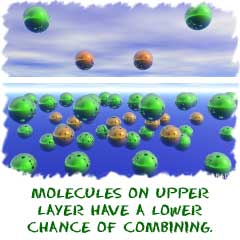 There is another big idea for rates of reaction called collision theory. The collision theory says that the more collisions in a system, the more likely combinations of molecules will happen. If there are a higher number of collisions in a system, more combinations of molecules will occur. The reaction will go faster, and the rate of that reaction will be higher.
There is another big idea for rates of reaction called collision theory. The collision theory says that the more collisions in a system, the more likely combinations of molecules will happen. If there are a higher number of collisions in a system, more combinations of molecules will occur. The reaction will go faster, and the rate of that reaction will be higher.
Reactions happen, no matter what. Chemicals are always combining or breaking down. The reactions happen over and over but not always at the same speed. A few things affect the overall speed of the reaction and the number of collisions that can occur.
 Concentration: If there is more of a substance in a system, there is a greater chance that molecules will collide and speed up the rate of the reaction. If there is less of something, there will be fewer collisions and the reaction will probably happen at a slower speed.
Concentration: If there is more of a substance in a system, there is a greater chance that molecules will collide and speed up the rate of the reaction. If there is less of something, there will be fewer collisions and the reaction will probably happen at a slower speed.
Temperature: When you raise the temperature of a system, the molecules bounce around a lot more (because they have more energy). When they bounce around more, they are more likely to collide. That fact means they are also more likely to combine. When you lower the temperature, the molecules are slower and collide less. That temperature drop lowers the rate of the reaction.
Pressure: Pressure affects the rate of reaction, especially when you look at gases. When you increase the pressure, the molecules have less space in which they can move. That greater concentration of molecules increases the number of collisions. When you decrease the pressure, molecules don't hit each other as often. The lower pressure decreases the rate of reaction.
 There is another big idea for rates of reaction called collision theory. The collision theory says that the more collisions in a system, the more likely combinations of molecules will happen. If there are a higher number of collisions in a system, more combinations of molecules will occur. The reaction will go faster, and the rate of that reaction will be higher.
There is another big idea for rates of reaction called collision theory. The collision theory says that the more collisions in a system, the more likely combinations of molecules will happen. If there are a higher number of collisions in a system, more combinations of molecules will occur. The reaction will go faster, and the rate of that reaction will be higher. Reactions happen, no matter what. Chemicals are always combining or breaking down. The reactions happen over and over but not always at the same speed. A few things affect the overall speed of the reaction and the number of collisions that can occur.
 Concentration: If there is more of a substance in a system, there is a greater chance that molecules will collide and speed up the rate of the reaction. If there is less of something, there will be fewer collisions and the reaction will probably happen at a slower speed.
Concentration: If there is more of a substance in a system, there is a greater chance that molecules will collide and speed up the rate of the reaction. If there is less of something, there will be fewer collisions and the reaction will probably happen at a slower speed. Temperature: When you raise the temperature of a system, the molecules bounce around a lot more (because they have more energy). When they bounce around more, they are more likely to collide. That fact means they are also more likely to combine. When you lower the temperature, the molecules are slower and collide less. That temperature drop lowers the rate of the reaction.
Pressure: Pressure affects the rate of reaction, especially when you look at gases. When you increase the pressure, the molecules have less space in which they can move. That greater concentration of molecules increases the number of collisions. When you decrease the pressure, molecules don't hit each other as often. The lower pressure decreases the rate of reaction.
Chemical Reactions
Let's start with the idea of a reaction. In chemistry, a reaction happens when two or more molecules interact and something happens. That's it. What molecules are they? How do they interact? What happens? Those are all the possibilities in reactions. The possibilities are infinite. There are a few key points you should know about chemical reactions.
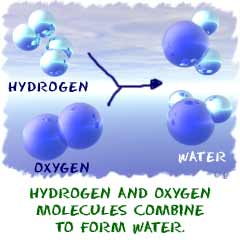
2. A reaction could include ions, molecules, or pure atoms. We said molecules in the previous paragraph, but a reaction can happen with anything, just as long as a chemical change occurs (not a physical one). If you put pure hydrogen gas (H2) and pure oxygen gas in a room, they can be involved in a reaction. The slow rate of reaction will have the atoms bonding to form water very slowly. If you were to add a spark, those gases would create a reaction that would result in a huge explosion. Chemists would call that spark a catalyst.
 3. Single reactions often happen as part of a larger series of reactions. Take something as simple as moving your arm. The contraction of that muscle requires sugars for energy. Those sugars need to be metabolized. You'll find that proteins need to move in a certain way to make the muscle contract. A whole series (hundreds actually) of different reactions are needed to make that simple movement happen.
3. Single reactions often happen as part of a larger series of reactions. Take something as simple as moving your arm. The contraction of that muscle requires sugars for energy. Those sugars need to be metabolized. You'll find that proteins need to move in a certain way to make the muscle contract. A whole series (hundreds actually) of different reactions are needed to make that simple movement happen.

Key Points
1. A chemical change must occur. You start with one compound and turn it into another. That's an example of a chemical change. A steel garbage can rusting is a chemical reaction. That rusting happens because the iron (Fe) in the metal combines with oxygen (O2) in the atmosphere. When a refrigerator or air conditioner cools the air, there is no reaction. That change in temperature is a physical change. Nevertheless, a chemical reaction can happen inside of the air conditioner.2. A reaction could include ions, molecules, or pure atoms. We said molecules in the previous paragraph, but a reaction can happen with anything, just as long as a chemical change occurs (not a physical one). If you put pure hydrogen gas (H2) and pure oxygen gas in a room, they can be involved in a reaction. The slow rate of reaction will have the atoms bonding to form water very slowly. If you were to add a spark, those gases would create a reaction that would result in a huge explosion. Chemists would call that spark a catalyst.
 3. Single reactions often happen as part of a larger series of reactions. Take something as simple as moving your arm. The contraction of that muscle requires sugars for energy. Those sugars need to be metabolized. You'll find that proteins need to move in a certain way to make the muscle contract. A whole series (hundreds actually) of different reactions are needed to make that simple movement happen.
3. Single reactions often happen as part of a larger series of reactions. Take something as simple as moving your arm. The contraction of that muscle requires sugars for energy. Those sugars need to be metabolized. You'll find that proteins need to move in a certain way to make the muscle contract. A whole series (hundreds actually) of different reactions are needed to make that simple movement happen.
Browsing Biochemistry...
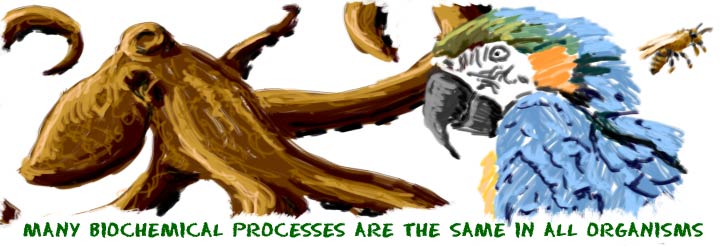 If you had visited Biology4Kids you may recognize the topics of this section. We felt it was more appropriate to have the biochemistry section here on Chem4Kids. It is one of the crossover fields of chemistry. Biochemists have to understand both the living world and the chemical world to be the best at their jobs.
If you had visited Biology4Kids you may recognize the topics of this section. We felt it was more appropriate to have the biochemistry section here on Chem4Kids. It is one of the crossover fields of chemistry. Biochemists have to understand both the living world and the chemical world to be the best at their jobs. The key thing to remember is that biochemistry is the chemistry of the living world. Plants, animals, single-celled organisms... They all use the same basic chemical compoundscycles to live their lives. Biochemistry is not about the cells or the organisms. It's about the smallest parts of those organisms, the molecules. It's also about the that happen to create those compounds.
Those cycles that repeat over and over are the things that allow living creatures to survive on Earth. It could be the constant process of photosynthesis in plants that creates sugars or the building of complex proteins in the cells of your body. Every cycle has a place and they are just one building block that helps organisms live. In each of those cycles, molecules are needed and changed. It's one big network of activity where each piece relies on all of the others.
6/14/09
chaiyokk...(from dragon sakura)

i can go on my way without getting lost, getting closer to my wishes,
i see the light wake up, stand up, and tomorrow try again,
look at my little hand, hold on tight, my precious,
my vision in me seems to disapear,
but i dont want to lose my way,
do you know that i want it all this dream continues to shine in my heart,
dont forget it,
i can go on my way without getting lost,
getting closer to my wishes,
one day, this flower full of ligth will blomm,
i will figth with all my strength,
i want to run like i'm runing today,
one day in the palce of our dreams,
we could laugh, wake up, stand up, and dont stop getting up and trying,
getting closer to my wishes,
one day, this flower full of ligth will blomm,
i will figth with all my strength,
i want to run like i'm runing today,
one day in the palce of our dreams,
we could laugh, wake up, stand up, and dont stop getting up and trying,
AK TERKOTANG KATIK.....
ntah pe2 je ak nie. asyik fobia je ngn final. almaklumlah 2 case dh berlaku kt diri ak mase final.
sape yg x trauma kn kn. ak pn dh x caye dh kt .................. menyakitkn je..disebabkn TRAGEDI PEDIH tu ak mgkn ak kehilangn 2 ORG TERSAYANG yg selama nie ak kagum,respect.....HUHUHUHUH
YA ALLAH, berilah ak kekuatan utk menempuh dugaan dan cabaran ini...
YA ALLAH, bekalknlah ak dgn kekuatan iman agar dpt ku mghadapinye...
YA ALLAH, lindungilah ak dr fitnah dan tohmahan dunia...
...YA ALLAH, YA RAHMAN, YA RAHIM...
Subscribe to:
Posts (Atom)





